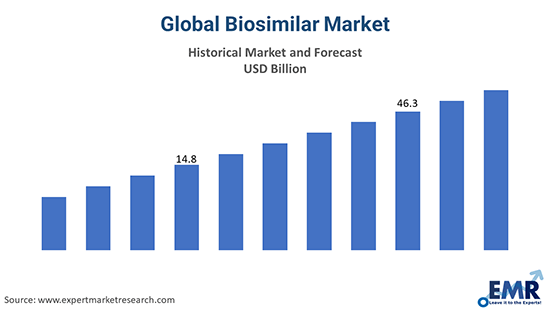
The biosimilar market has emerged as one of the fastest-growing segments in the pharmaceutical industry, driven by the rising demand for cost-effective biologic alternatives. Global Biosimilar Market With the expiration of patents for blockbuster biologics and the increasing prevalence of chronic diseases, biosimilars are gaining traction as a key solution to reduce healthcare expenditure while maintaining therapeutic efficacy. As the market evolves, stakeholders are focusing on innovation, regulatory compliance, and strategic partnerships to capitalise on emerging opportunities.
Get a Free Sample Report with a Table of Contents: https://www.expertmarketresearch.com/reports/biosimilar-market/requestsample
Biosimilars are biologic medical products highly similar to already approved reference biologics. Global Biosimilar Market They offer equivalent safety, efficacy, and quality while being more affordable, addressing the global need for accessible healthcare solutions. The market encompasses a wide range of therapeutic applications, including oncology, autoimmune diseases, and diabetes, reflecting its significant role in addressing critical healthcare challenges.
North America and Europe dominate the global biosimilar market, benefiting from supportive regulatory frameworks and robust healthcare infrastructure. However, the Asia-Pacific region is rapidly emerging as a lucrative market due to increasing healthcare spending, a growing patient pool, and the entry of domestic players.
Drivers
-
Patent Expirations of Biologic Drugs: The expiry of patents for several high-revenue biologics is paving the way for biosimilar development, driving market growth.
-
Rising Prevalence of Chronic Diseases: The growing burden of cancer, autoimmune disorders, and diabetes is fueling demand for biosimilars as cost-effective treatment options.
-
Cost Savings for Healthcare Systems: Biosimilars offer significant cost advantages, enabling governments and healthcare providers to allocate resources more efficiently.
-
Supportive Regulatory Environment: Streamlined approval pathways in regions like the EU and the US are accelerating market entry for biosimilar products.
Read Full Report with Table of Contents: https://www.expertmarketresearch.com/reports/biosimilar-market
Challenges
-
Complex Manufacturing Processes: The production of biosimilars involves sophisticated technology and stringent quality controls, posing challenges for manufacturers.
-
Market Penetration Barriers: Physician scepticism and limited awareness among patients can hinder adoption in some markets.
-
Regulatory Hurdles in Emerging Markets: Inconsistent regulatory frameworks in developing countries can delay product launches.
Opportunities
-
Expansion in Emerging Markets: Increasing healthcare expenditure and rising awareness in Asia-Pacific, Latin America, and the Middle East provide significant growth potential.
-
Advancements in Biomanufacturing: Innovations in production technologies are reducing costs and improving the scalability of biosimilar manufacturing.
-
Strategic Collaborations: Partnerships between pharmaceutical companies and research organisations are fostering innovation and accelerating market growth.
Rising Demand for Oncology Biosimilars
With cancer being a leading cause of mortality worldwide, the demand for oncology biosimilars is growing rapidly. These products are becoming integral to treatment regimens, offering effective solutions at reduced costs.
Increasing Investment in R&D
Major pharmaceutical companies are investing heavily in biosimilar research and development, focusing on expanding their product portfolios and improving production efficiency.
Growing Focus on Digital Health Integration
The integration of digital health technologies is enhancing patient adherence, monitoring, and outcomes, particularly in chronic disease management involving biosimilars.
Market Consolidation
Mergers and acquisitions among key players are reshaping the competitive landscape, enabling companies to strengthen their market presence and capabilities.
By Product Type
-
Monoclonal Antibodies: Widely used for treating cancer, autoimmune diseases, and inflammatory conditions.
-
Insulin Biosimilars: Address the growing prevalence of diabetes worldwide.
-
Erythropoietin Biosimilars: Used for treating anaemia associated with chronic kidney disease and cancer.
-
Granulocyte Colony-Stimulating Factors (G-CSF): Support patients undergoing chemotherapy by boosting white blood cell production.
By Indication
-
Oncology: Accounts for the largest market share due to the rising incidence of cancer.
-
Autoimmune Diseases: Includes conditions like rheumatoid arthritis, psoriasis, and inflammatory bowel disease.
-
Diabetes: Reflects the increasing global burden of diabetes and the need for affordable insulin alternatives.
By End-User
-
Hospitals: Primary users of biosimilars for inpatient care.
-
Clinics: Focused on outpatient treatment and chronic disease management.
-
Pharmacies: Drive retail sales of biosimilars for self-administration by patients.
By Region
-
North America: Dominates the market with high adoption rates and favourable reimbursement policies.
-
Europe: A mature market with well-defined regulatory pathways.
-
Asia-Pacific: Fastest-growing region due to increasing healthcare access and domestic manufacturing capabilities.
-
Latin America and Middle East & Africa: Emerging markets with untapped potential for biosimilar adoption.
The biosimilar market is expected to witness exponential growth due to:
-
Favourable Regulatory Pathways: Streamlined approval processes in developed regions are encouraging biosimilar adoption.
-
Increasing Healthcare Expenditure: Governments and private players are investing in biosimilar manufacturing to address affordability challenges.
-
Technological Advancements: Innovations in bioprocessing technologies are enhancing production efficiency and quality.
-
Rising Awareness Campaigns: Efforts by governments and NGOs are improving patient and physician acceptance of biosimilars.
-
Novartis AG: Expanded its biosimilar portfolio with the launch of a new oncology biosimilar in the US and Europe.
-
Orion Pharma: Announced strategic collaborations to enhance its presence in emerging markets, focusing on autoimmune disease biosimilars.
-
Pfizer Inc.: Strengthened its position in the market with successful clinical trials for a biosimilar targeting chronic inflammatory diseases.
The global biosimilar market encompasses a diverse range of therapeutic applications, addressing the needs of patients with chronic and life-threatening conditions. The integration of advanced manufacturing technologies, regulatory harmonisation, and growing public-private partnerships is expanding the scope of the market. Additionally, the increasing focus on personalised medicine and the development of biosimilar versions of high-value biologics highlight the market's potential to reshape global healthcare systems.
The COVID-19 pandemic had a mixed impact on the biosimilar market:
-
Positive Effects:
-
Increased focus on affordable healthcare solutions highlighted the importance of biosimilars.
-
Accelerated adoption of telemedicine and e-commerce channels facilitated patient access to biosimilars.
-
-
Negative Effects:
-
Disruptions in clinical trials and supply chains delayed product launches.
-
Reduced hospital visits during lockdowns temporarily impacted biosimilar sales.
-
Post-pandemic recovery is marked by resumed clinical activities, improved supply chain resilience, and increased investments in biosimilar development.
Novartis AG
A global leader in biosimilar development, Novartis AG focuses on oncology, immunology, and endocrinology biosimilars, leveraging its extensive R&D capabilities and strong market presence.
Orion Pharma
Known for its innovative approach, Orion Pharma is expanding its biosimilar portfolio, particularly in autoimmune diseases and oncology, targeting emerging markets.
Pfizer Inc.
Pfizer Inc. continues to strengthen its biosimilar offerings, with a focus on addressing unmet medical needs through high-quality, affordable alternatives to reference biologics.
FAQs
1. What is driving the growth of the biosimilar market?
The expiration of biologic drug patents, rising prevalence of chronic diseases, and demand for cost-effective healthcare solutions drive market growth.
2. Which regions dominate the biosimilar market?
North America and Europe lead the market due to supportive regulatory environments and advanced healthcare infrastructure.
3. How did COVID-19 impact the biosimilar market?
The pandemic disrupted clinical trials and supply chains but highlighted the importance of affordable healthcare solutions, boosting long-term market prospects.
4. Who are the major players in the biosimilar market?
Key players include Novartis AG, Orion Pharma, and Pfizer Inc., known for their innovation and strong market presence.
5. What are the key trends in the biosimilar market?
Key trends include the rising demand for oncology biosimilars, advancements in biomanufacturing, and increasing investment in R&D.