Abstract
This study investigated the metabolic activity and survival of five lactic acid bacteria strains in citrus flavanone standards. The results showed that L. plantarum N13 and L. brevis LB01 could metabolize hesperetin-7-O-rutinoside, while L. plantarum N13, L. acidophilus LA85, and L. brevis LB01 could metabolize naringenin-7-O-rutinoside. L. acidophilus LA85 exhibited the highest biotransformation ratio of naringenin-7-O-rutinoside. L. acidophilus LA85 and L. plantarum N13 could degrade naringenin-7-O-rutinoside into naringenin, while L. brevis LB01 could degrade hesperetin-7-O-rutinoside into hesperidin and other metabolites. The study suggests that L. acidophilus LA85 may contribute to the bioavailability of citrus flavanones and could be used as functional cultures to enhance bioactive metabolites in food or the gastrointestinal tract.
Introduction
Flavonoids are plant-based compounds known for their health benefits, but they're often poorly absorbed by the body. Microbial processes can transform them into more bioavailable forms. Citrus fruits are rich in flavanones, and lactic acid bacteria (LAB) can convert them into more useful compounds. This study looked at five LAB strains and their ability to transform citrus flavanones, potentially enhancing their health benefits.
Results
1. Effect of flavanones on the growth of LAB strains.
Figure 1 illustrates the effect of 20 mg/L concentrations of hesperetin-7-O-rutinoside or naringenin-7-O-rutinoside on the growth of L. acidophilus LA85, L. brevis LB01, L. plantarum N13, L. casei LC89, and L. rhamnosus LRa05. The OD620 value, reflecting both viable and dead bacteria, was monitored.
During the 72-hour period, the OD620 value of L. plantarum N13 steadily increased, indicating continuous growth. In contrast, for L. rhamnosus LRa05 and L. casei LC89, the OD620 value remained relatively stable at 48 and 72 hours (p > 0.05), but significantly decreased compared to 24 hours (p < 0.05). This suggests a cessation of growth likely due to carbon source depletion and subsequent autolysis.
Importantly, no significant differences in growth were observed among the lactic acid bacteria strains after 72 hours of exposure to MRS medium containing hesperetin-7-O-rutinoside or naringenin-7-O-rutinoside compared to untreated MRS medium. This indicates that all five LAB strains were resistant and able to grow in the presence of approximately 20 mg/L of orange juice flavanones over the 72-hour incubation period.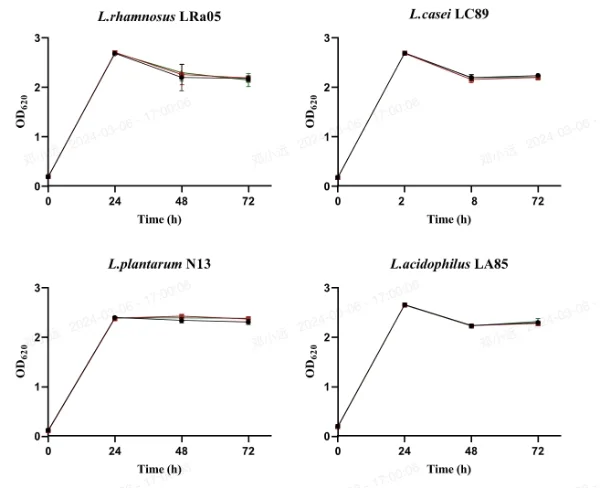
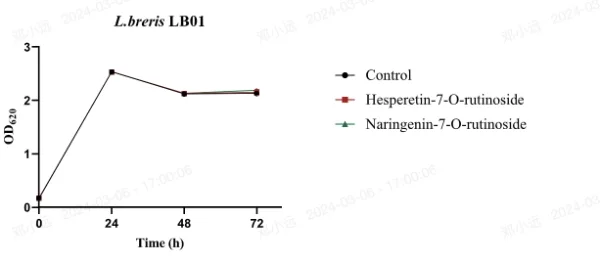
Fig. 1 Survival of LAB strains in MRS medium (Control), 20 mg/L of hesperetin-7-O-rutinoside or naringenin-7-O-rutinoside solutions (mean ± standard deviation) of OD620 (n=3)
2. β‑Glucosidase activity
As can be seen from Table 1, L. rhamnosus LRa05 was the strain with the highest β-glucosidase activity, while the other four stains exhibited similar β-glucosidase activity. The results indicated that the five LAB strains applied in the experiment all possessed β-glucosidase activity, which were suitable for further study on the biotransformation of hesperetin-7-O-rutinoside or naringenin-7-O-rutinoside. No α-rhamnosidase activity were detected of the five strains (data not shown).
Table 1 β-Glucosidase activity of probiotic bacteria Lactobacillus acidophilus LA85, L. brevis LB01, Lactobacillus plantarum N13, Lactobacillus casei LC89, and L. rhamnosus LRa05
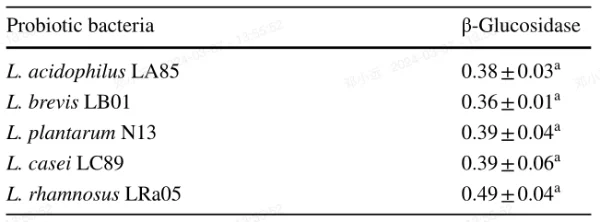
Activity of β-Glucosidase expressed as units of enzyme activity
One unit of enzyme activity is the amount of enzyme that released 1 nmol of p-nitrophenol from the substrate per mL per min at 37 ℃. The data expressed in mean ± standard deviation (n=3),a Means significant differences of enzyme production ability among different strains (p<0.05)
3. Antioxidant and α‑glucosidase inhibition properties before and after biotransformation
To assess potential antioxidant activities contributed by the bacteria, we evaluated the DPPH and ABTS radical scavenging activities of a standard flavanone solution incubated with the LAB strain. Table 2 shows that there were no significant changes (p > 0.05) in the DPPH and ABTS radical scavenging activities of the naringenin-7-O-rutinoside solution after 72 hours of incubation with L. acidophilus LA85.
Additionally, we investigated the α-glucosidase inhibition rate, a marker for postprandial hyperglycemia, in Table 2. There were no significant differences (p > 0.05) in the α-glucosidase inhibition rate between 0 and 72 hours of incubation of naringenin-7-O-rutinoside with L. acidophilus LA85.
In summary, incubation of L. acidophilus LA85 in naringenin-7-O-rutinoside solution did not appear to influence the tested biological activities.
Table 2 Effect of incubated with L. acidophilus LA85 on the in vitro activities of naringenin-7-O-rutinoside
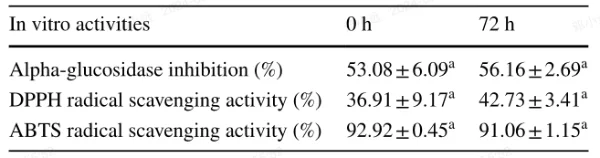
The data expressed as mean ± standard deviation (n=3). Letter in the same row indicate significant differences of the in vitro activity between 0 h and 72 h (p<0.05)
Conclusions
This study found that metabolites produced by LAB in flavanone standard solutions did not affect antioxidant and α-glucosidase inhibition properties. Specifically, L. acidophilus LA85 demonstrated enzymatic activity capable of degrading naringenin-7-O-rutinoside to naringenin by cleaving glycosidic bonds. Therefore, L. acidophilus LA85 may contribute to the bioavailability of orange juice flavanones. In contrast, L. rhamnosus LRa05, L. casei LC89, L. plantarum N13, and L. brevis LB01 showed low activity in flavanone biotransformation, although L. brevis LB01 exhibited potential for producing active metabolites.
The selection of strains for flavonoid biotransformation is crucial. Screening for strains that can efficiently metabolize citrus flavanones may lead to the development of functional cultures that produce more bioavailable and bioactive metabolites in food products or the human gastrointestinal tract. However, further research is needed to fully understand the effects of these bacteria on flavanone metabolism.
Wecare Probiotics will continue to closely monitor research related to probiotics and lactic acid bacteria, striving to enhance our understanding and application of these beneficial microorganisms. Our commitment is to advance the field of probiotics, contributing to improved health and well-being through innovative solutions.
